The SI joint can be a significant cause of lower back pain. Clinical publications have identified the SI joint as a pain generator in 15-30% of chronic lower back pain patients.1-4 In addition, the SI joint is a pain generator in up to 43% of patients with continued or new onset lower back pain after a lumbar fusion.5
Do you have SI Joint Pain?
Sacroiliac Joint (SI Joint) Anatomy
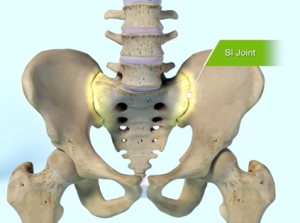
The SI joint is the link between the ilium, in the pelvis, to the sacrum, which is the lowest part of the spine above the tailbone.
The sacroiliac joint (SI joint) is located in the pelvis; it links the iliac bones (pelvis) to the sacrum (lowest part of the spine above the tailbone). It is an essential component for energy transfer between the legs and the torso.
Like any other joint in the body, the SI joint can be injured and/or undergo degeneration. When this happens, people can feel pain in their buttock and sometimes in the lower back, hips and legs. This is especially true while lifting, running, walking or even lying on the involved side.
Dysfunctional SI Joint
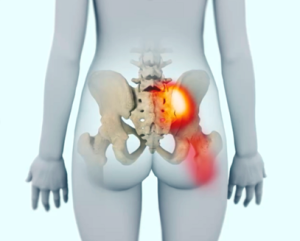
A dysfunctional SI joint may inflame the joint and surrounding nerves causing pain in the lower back, hip, groin, or pelvis.
A dysfunctional SI joint may inflame the joint and surrounding nerves causing pain in the lower back, hip, groin, or pelvis.
It’s common for pain from the SI joint to feel like disc or lower back pain, or sometimes hip or groin pain. For this reason, SI joint disorders should always be considered in lower back, hip, and pelvic pain diagnosis.
Do you experience one or more of the symptoms listed below?
- Lower back pain
- Sensation of low extremity: pain, numbness, tingling, weakness
- Pelvis/buttock pain
- Hip/groin pain
- Feeling of leg instability (buckling, giving way)
- Disturbed sleep patterns due to pain
- Disturbed sitting patterns (unable to sit for long periods, sitting on one side)
- Pain going from sitting to standing

Stepping Up
SI joint pain can mimic pain in the lower back, hip, groin, or pelvis.

Sit to Stand
Patients who suffer from SI joint dysfunction can have severe pain when performing transitional movements like standing from a chair.

Painful Sitting
Patients who have SI joint pain usually find it difficult to sit for long periods of time, and usually try to alleviate the discomfort by sitting on the least effected side.
Making a Diagnosis
A variety of tests performed during physical examination may help reveal the SI joint as the cause of your symptoms. Sometimes, X-rays, CT-scan or MRI may be helpful in the diagnosis of SI joint-related problems because they can rule out other common sources of pain—such as your lumbar spine or hip joints. It is also important to remember that other conditions (like a disc problem) can co-exist with SI joint disorders.
The most relied upon method to accurately determine whether the SI joint is the cause of your lower back pain symptoms is to inject the SI joint with a local anesthetic. This diagnostic injection will be performed under either X-ray or CT guidance to verify accurate placement of the needle in the SI joint. If your symptoms decrease by at least 75%, it can be concluded that the SI joint is either the source of or a major contributor to your lower back, hip, or pelvic pain. If the level of pain does not change after SI joint injection, it is less likely that the SI joint is the cause of your pain.
Treatment Options
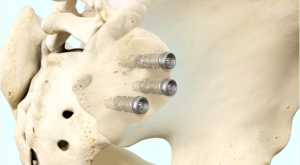
The iFuse TORQ Implant System typically involves placing three threaded titanium implants across the SI joint to maximize stability.
Once the SI joint is confirmed as the cause of your symptoms, treatment can begin. Some patients respond well to physical therapy, use of oral medications, or injection therapy. These treatments are often performed repetitively, and frequently symptom improvement using these therapies is temporary. If non-surgical treatment options have been tried and do not provide long-term relief, your surgeon may consider other options, including the minimally invasive iFuse procedure.
SI Joint Fusion with iFuse TORQ® Implant System
Option #1 Treatment
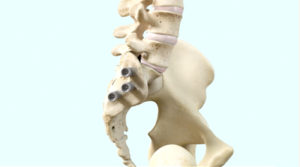
The iFuse TORQ Implant System is designed to provide stabilization and fusion for certain SI joint disorders.
iFuse TORQ® is designed to stabilize and fuse the SI joint. The iFuse procedure involves inserting typically three threaded titanium implants across the SI joint to maximize stability, reduce pain, and improve function. The procedure is done through a small one-inch incision and takes about an hour. The 3D-printed iFuse TORQ implant was designed for osseointegration, which is the structural and functional connection between implant and bone. This allows your painful joint to be stabilized through binding of bone all along the implant.6
iFuse Technology®

The 3D printed surface of iFuse TORQ is similar to cancellous bone and has the same iFuse Technology® used in SI-BONE’s original triangular, titanium implants.
iFuse TORQ® Implant System is one of the latest innovative solutions from SI-BONE, the creator of the minimally-invasive SI joint fusion device—the triangular titanium iFuse Implant. More than 100, peer-reviewed publications demonstrate the safety, durable effectiveness, and biomechanical and economic benefits of the iFuse implant (www.si-bone.com/results). The iFuse implant is the only SI joint fusion device with multiple prospective clinical studies, including two randomized controlled trials7,8, demonstrating that treatment improved pain, patient function, and quality of life.7-12 As with any minimally invasive surgical procedures, there are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit www.si-bone.com/risks.
SIJ: Where it is and how does it work?
SIJ Dysfunction: Prevalence & Causes
iFuse TORQ: Minimally Invasive SI joint fusion
References
- Bernard TN, et al. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;217:266–80.
- Schwarzer AC, et al. The Sacroiliac Joint in Chronic Low Back Pain. Spine. 1995;20:31–7.
- Maigne JY, et al. Results of Sacroiliac Joint Double Block and Value of Sacroiliac Pain Provocation Tests in 54 Patients with Low Back Pain. Spine. 1996;21:1889–92.
- Sembrano JN, et al. How Often is Low Back Pain Not Coming From The Back? Spine. 2009;34:E27–32.
- DePalma MJ, et al. Etiology of Chronic Low Back Pain Patients Having Undergone Lumbar Fusion. Pain Med. 2011;12:732-9.
- SI-BONE 300857-R.
- Polly DW, et al., and the INSITE Study Group. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016;10:Article 28. DOI: 10.14444/3028
- Dengler J, et al. Randomized Trial of Sacroiliac Joint Fusion vs. Conservative Management for Chronic Low Back Pain Attributed to the Sacroiliac Joint. J Bone Joint Surg Am. 2019;101(5):400-11. DOI: 10.2106/JBJS.18.00022.
- Duhon B, Bitan F, Lockstadt H, Kovalsky D, Cher D, Hillen T, on behalf of the SIFI Study Group. Triangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: 2-Year Follow-Up from a Prospective Multicenter Trial. Int J Spine Surg. 2016;10:Article 13. DOI: 10.14444/3013
- Dengler J, et al. on behalf of the INSITE, iMIA and SIFI study groups. Predictors of Outcome in Conservative and Minimally Invasive Surgical Management of Pain Originating from the Sacroiliac Joint – a Pooled Analysis. Spine. 2017;42(21):1664-73. [Epub 2017 Mar 27]. DOI: 10.1097/BRS.0000000000002169
- Whang PG, et al. Long-Term Prospective Clinical and Radiographic Outcomes After Minimally Invasive Lateral Transiliac Sacroiliac Joint Fusion Using Triangular Titanium Implants. Med Devices (Auckl). 2019;12:411-422. DOI: 10.2147/MDER.S219862
- Patel V, et al. Prospective Trial of Sacroiliac Joint Fusion Using 3D-Printed Triangular Titanium Implants: 24-Month Follow-Up. Med Devices (Auckl). 2021;14:211-216. DOI: 10.2147/MDER.S314828
The iFuse TORQ® Implant System is indicated for:
- Fusion of the sacroiliac joint for sacroiliac joint dysfunction including sacroiliac joint disruption and degenerative sacroiliitis.
- Augmenting immobilization and stabilization of the sacroiliac joint in skeletally mature patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion.
The iFuse TORQ Implant System is also indicated for fracture fixation of the pelvis, including acute, non-acute, and non-traumatic fractures.
The iFuse TORQ Navigation instruments are intended to be used with the iFuse TORQ Implant System to assist the surgeon in precisely locating anatomical structures in iFuse TORQ Implant System procedures, in which the use of stereotactic surgery may be appropriate, and where reference to a rigid anatomical structure, such as the pelvis or vertebra, can be identified relative to the acquired image (CT, MR, 2D fluoroscopic image or 3D fluoroscopic image reconstruction) and/or an image data based model of the anatomy. iFuse TORQ Navigation instruments are intended to be used with the Medtronic® StealthStation® System.
There are potential risks associated with the iFuse procedures. It may not be appropriate for all patients and all patients may not benefit. For information about the risks, visit www.si-bone.com/risks.


